War in the Womb: The Evolution of Pre-eclampsia
The most common serious pregnancy complication arises due to an ancient conflict between mothers and fathers
First published in on Medium

It could have ended badly in so many ways. My friend, let’s call her Amy, in vibrant mid-twenties health, could not have been more ready for her first pregnancy. Apart from some nausea in the first trimester, she breezed through the first eight months. As her due date approached, however, Amy’s hands, feet, and face puffed up with retained fluid. A Friday afternoon visit to her doctor showed high blood pressure and some protein in her urine.
By early the next week, Amy’s face had swollen so much that her eyes were almost shut, her blood pressure had rocketed to 160 over 110, and her urinary protein levels spelled a warning. Doctors watch for these symptoms because they often portend the most common dangerous pregnancy complication: preeclampsia.
The only known cure for preeclampsia is to deliver the baby. With only two weeks until Amy’s due date, the obstetrician didn’t hesitate to induce labor. After many long hours, a scare when the baby’s heart rate began to drop, and a dramatic forceps delivery, Amy lapsed into convulsions. Pre-eclampsia progressed to eclampsia, a mortally dangerous condition.
With modern medical care, fewer than one in 200 women with preeclampsia progress to eclampsia. Among those who do, many sustain massive damage to their kidneys, liver, or brain. Quite a few die.
Between three and ten percent of pregnancies are classed as pre-eclamptic, although their symptoms seldom match Amy’s for drama. Yet, despite its high rate of incidence and the considerable suffering it causes, science knows remarkably little about why pre-eclampsia occurs.
Evolution
The causes of pre-eclampsia remain so murky that it is often called “a disease of theories”
As an evolutionary biologist, my job is to pose — and to attempt to answer — questions that begin with “Why?”. Questions like “Why are there two sexes?”; “Why do our bodies deteriorate as we get old?”; and “Why do our bodies sometimes not work sensibly, developing conditions like pre-eclampsia?”
Other branches of science ask different kinds of questions, equally valid and often more pressing ones. Like “How does pre-eclampsia progress?”, “ What combination of symptoms best predict which pregnancies will become pre-eclamptic?” And “How should hospital staff treat a pregnant woman who presents with those symptoms?” Answering these “How?” questions leads to cures and management plans that save lives.
And yet we often remain no closer to a deep understanding of why our bodies work – or malfunction – in the ways that they do. The causes of preeclampsia remain so murky that it is often called “a disease of theories.”
The evolutionary conflict theory of pre-eclampsia’s origins is a sophisticated, multi-faceted beauty. It is built on the foundation of two under-appreciated facts. First, even our most profoundly loving relationships — between mother and child and between mother and father — bristle with conflicting evolutionary interests. Second, a father is not always the biological parent of a child born to his partner.
Placenta of attention
Before we can get to these deep motives, I should tell you that we already have a suspect. Whatever pre-eclampsia’s evolutionary causes, the placenta lurks in the background like the suspect in a crime novel. Like many suspects, the fleshy lump of membranes and blood vessels embodies more substance than style. It might not be much to look at, but the human placenta ranks among the most exquisitely functional organs that ever evolved.
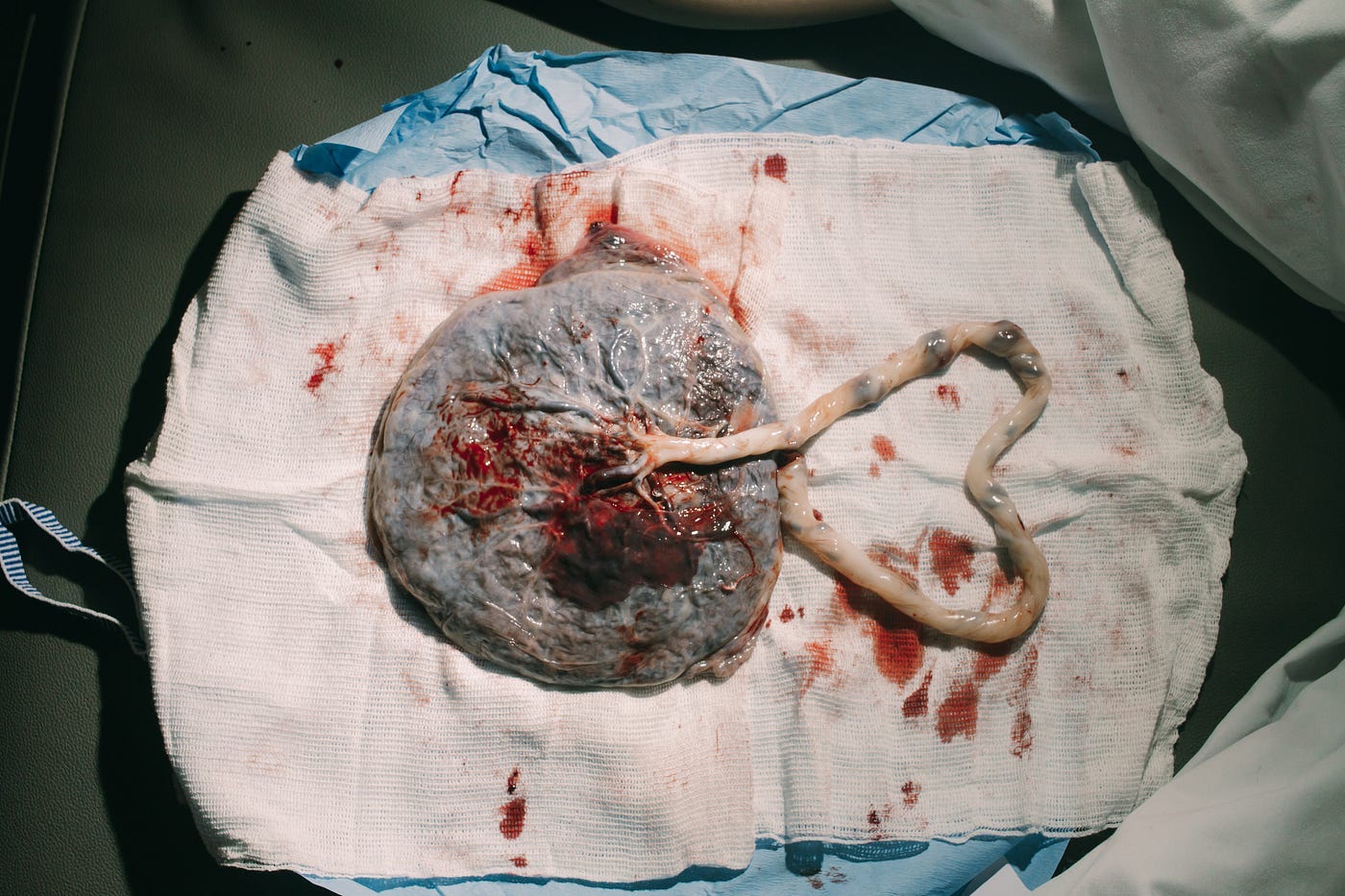
Roughly a week after a male’s sperm finds and fertilizes an egg, the resulting embryo nestles into the endometrium, a velvety carpet of blood vessels lining the mother’s uterus. Within days, cells from the embryo invade the endometrium like the first shallow roots of a germinated seed growing into the soil. These root-like outgrowths become the placenta. Four weeks into pregnancy, the umbilical cord connecting the placenta to the embryo is visible, and the heart starts beating.
A membrane no wider than the skin of a soap bubble separates the fetus’ blood from the mother’s. Oxygen, nutrients, and antibodies perfuse from mother to fetus. Waste products travel the other way, to be disposed of by the mother’s liver, kidneys, and lungs.
The placenta makes hormones that maintain the endometrium and ensure the placenta stays attached. The hormones also regulate the amount of glucose, fat, and protein in the mother’s bloodstream and, thus, the nutrients available to the fetus. In so many ways, the placenta mediates affairs between a pregnant mother and the new individual growing inside her.
It seems only fair, then, to venerate the placenta as one of the body’s unsung heroes. For nine months, it brokers a mother’s devotion to her darling, dependent offspring. People in many cultures revere the placenta, burying it in a sacred place. In a few societies – and some hippy circles – mothers even eat the placenta. Yet, viewing the human placenta as a peaceful intermediary working harmoniously for the good of both mother and her precious, growing progeny ignores a more intriguing and conflicted reality.
From the perspective of a pregnant woman’s body, both the placenta and fetus are invaders. They intrude, like semi-welcome house guests, and take more than their polite share from the host. From the moment a developing embryo implants in the mother’s uterus, fetal cells set to work remodeling the mother’s arteries, limiting her control over blood flow to the placenta.
A human mother cannot reduce the nutrients the placenta receives without lowering the overall concentration of those nutrients in her own blood. But the placenta can release hormones directly into her bloodstream, instructing her body to give the placenta and fetus what they want. These features give the fetus considerable control over the mother’s blood pressure and the nutrient content of that blood.
In mother-offspring negotiations, therefore, the human fetus has a decisive advantage: the placenta acts as the fetus’ agent. Or, as the reproductive physiologist E.W. Page put it way back in 1939, the placenta might better be viewed as
a ruthless parasitic organ existing solely for the maintenance and protection of the fetus, perhaps too often to the disregard of the maternal organism.
The nagging fetus
An unborn fetus manipulates its mother into providing more of whatever the fetus wants
Harvard evolutionary theorist David Haig certainly sympathizes with this view. Over the last 25 years, Haig’s ideas have rewritten our understanding of the relationship between mother and fetus. Instead of viewing gestation as a benign process to safely grow babies, Haig’s ideas reveal a womb that simmers with conflict.

Imagine a fetus that can persuade its mother to circulate a little more glucose in the blood than she otherwise would. Or perhaps a fetus that can trick its mother into raising her blood pressure. Higher blood pressure pushes more glucose, oxygen, and other nutrients across the placental membranes and into the fetus’ bloodstream. What I’m asking you to imagine, in a word, is a fetus that can nag. Exactly like a baby crying for more milk or a toddler begging for a treat, an unborn fetus manipulates its mother into providing more of whatever the fetus wants.
If the mother delivers a high-pressure flow of glucose-rich blood to the placenta, the fetus will grow well and thrive. If she does not, the baby will be born small, with weaker survival prospects. Fetuses have evolved ways of persuading their mothers to increase their blood glucose and to elevate their blood pressure. Fetuses that successfully do so grow to be bigger and healthier at birth than less persuasive fetuses.
The most successful mothers in our evolutionary past were those that not only carried each baby safely to term but those that could then recover from childbirth and breastfeeding and do it all again. Pregnancy and breastfeeding exhaust a mother and sap her energy reserves. And for most of history, food was so scarce that mums struggled to eat for one, let alone for two.
Mothers who were taxed too much during pregnancy and breastfeeding by a demanding baby had less energy to care for their older children and took longer to recover and have another child. The baby might suffer a little from having a tired mother, but the mother would lose a lot more, in evolutionary terms, if her exhaustion limited the number of children she could bear. As a result, a mother should resist the nagging of her selfish unborn fetus and its ally, the placenta, providing just enough nutrients for the fetus to be born healthy.

This notion of pregnancy as a battle of physiological wills, between nagging fetus and resistant mother, undermines many cherished certainties. We can no longer look upon a pregnant mother as a benign Madonna-like incubator for an innocent new life. Without a change in perspective, it can be hard to understand why a mother and her offspring should battle one another so brutally. Surely both lose out if the mother suffers harm or if the baby doesn’t grow big enough?
To resolve the evolutionary paradox of mother-fetus conflict, it helps to imagine a gene’s point of view — rather than the individual’s — involved. Evolutionary theorists find the strange mental trick of imagining a gene’s evolutionary interests incredibly useful. That does not mean we are imputing consciousness on pieces of DNA. Instead, it allows us to imagine what kinds of genes might have arisen in the past and been successfully passed down from ancestor to descendant. They are the kinds of genes that made our ancestors successful at becoming parents, grandparents, aunties, and uncles.
David Haig employed this method to imagine the evolutionary interests of the genes involved in pregnancy. A mother’s genes benefit from a successful pregnancy that ends in a healthy baby, but her genes benefit equally from future successful pregnancies and healthy babies. It takes more effort to imagine the interests of the fetus’ genes because half of those genes come from the mother and the other half from the father.
The genes from mum do well, in evolutionary terms, if the offspring survives and thrives. But they also benefit if the mother recovers quickly from her pregnancy and has many more children. That is where the relationship between mother and fetus gets complicated because a gene that the fetus inherited from its mother will only be present in half of the mother’s other children. That gene has more to gain from the fetus’ success than from the success of its siblings. So we expect that natural selection would have favored some genes that allow the fetus to take more than the mother wants to give, as long as the harm to the mother is not too great.
The other half of a fetus’ genes, those that come from the father, should have even less compunction about harming the mother. And here, the fact that, throughout evolution, humans rarely mated for life becomes important. The genes that a fetus gets from its father have a smaller chance of being shared with other children by the same mother because there is a chance another man sired those other children. As a result, the genes that a fetus gets from its father don’t depend all that much on whether the mother flourishes. Haig argued that paternally-inherited genes should side decisively with the fetus and against the mother, taking her for all she can give.
Genes that opt-out
Each of us inherits one copy of each gene from each parent. In most cases, both copies get turned on — biologists say expressed — to make the body work. In some cases, only the copy that comes from the mother is expressed. For other pairs of these imprinted genes, only the paternal copy is expressed, and the maternal copy is silenced.
Geneticists disagree, often quite robustly, about why genes imprint. Only some plant and animal groups have evolved this trick. Reptiles, birds, and the egg-laying platypus don’t have imprinted genes, so it appears that imprinting in mammals evolved along with the placenta. Many of the genes that imprint are somehow involved in pregnancy and fetal growth. According to David Haig’s theory, that’s because of the conflicts that pit the genes the fetus inherits from the father against those from the mother.
Silencing allows genes to ‘opt-out’ of doing something that would harm the interests of the parent from whom they came. When the maternal copy of a gene is silenced, it is opting out of doing something that would harm the mother, like pestering her for more nutrients delivered at higher blood pressure. Silenced paternal genes are usually opting out of defending the mother against a rapacious fetus.
The hormone Insulin-like Growth Factor II (IGF2) promotes the invasion of the placenta into the uterine lining and the proliferation of cells in many tissues of the body. It is precisely the kind of hormone that favors the fetus as it battles the mother for resources. As we might predict, it is the embryo’s paternal copy of the Igf2 gene that manufactures IGF2. The copy inherited from the mother is silenced, opting out of doing something that might damage the mother.
But mothers have a defense against IGF2: the H19 gene, which soaks up excess growth factor, preventing the embryo from invading too far and the fetus from growing too rapidly. The father’s copy of H19 is silenced, as you might predict for a gene that benefits the mother by limiting the fetus’ growth.
When a tiny biochemical mistake fails to silence the maternal copy of Igf2, the embryo makes too much IGF2. When the maternal H19 gene doesn’t work properly, it is unable to restrain IGF2. In both cases, newborns suffer from Beckwith-Wiedemann syndrome: enormous birth weight, a high susceptibility to cancers, and low blood sugar after birth.
Profound as the problems caused by mistakes in imprinted genes are, they are also rare. But they illustrate how conflicts between mother and fetus can give rise to more common afflictions, like pre-eclampsia.
Not invasive enough

In the very early days of pregnancy, the conflict between the interests of the mother and fetus already begins simmering. For the fetus to flourish, the placenta must invade deep into the endometrium of the uterus and remodel blood vessels called spiral arterioles. The mother, however, benefits if she can limit the placenta’s invasion and retain some control over the blood flow to the placenta.
Human fetuses require such deep invasion of the placenta that the mother’s immune system must make serious compromises to tolerate the fetal tissue. To the mother, an embryo attempting to implant is both a cherished potential offspring and a nasty invader from the outside world. University of Winnipeg biologist Scott Forbes calls the placenta a ‘natural born cancer’ — a tumor whose invasion into the uterus must be contained and whose growth must be restricted for the term of the pregnancy.
The tussle over how deeply the placenta invades early in the first trimester affects the chances of pre-eclampsia much later on, toward the end of the third trimester. The mother’s immune responses to the implanting embryo are known to affect the chances of pre-eclampsia. An embryo’s success in implanting and then invading depends on how that embryo gets along with the natural killer cells of the mother’s immune system.
Several identified sets of genes, including the IGF2/H19 genes, also affect how deeply the placenta can invade. If the mother wins, the placenta might not invade deeply enough into the endometrium, failing to alter the mother’s blood vessels sufficiently. For the first two trimesters of pregnancy, when the fetus’ needs are modest, this makes little difference. But late in the pregnancy, when the fetus’ needs start to tax the mother’s body, not enough oxygen reaches the fetus to nourish its growing body and especially its oxygen-hungry brain.
A deadly tantrum
The panicked fetus and the placenta throw a massive tantrum to get what they need.
Throughout pregnancy, the fetus and placenta bicker with the mother over her blood pressure. The balance of power rests with the mother early on, while both fetus and placenta are small. She keeps her blood pressure low and prevents the fetus from growing too fast. But as the fetus grows, the power shifts, and team fetus-placenta starts to get its way. As the third trimester progresses, the mother’s blood pressure creeps skyward.

Every mother and fetus wage their private battle, but most fetuses receive what they need in order to grow. In pregnancies where the placenta hasn’t invaded sufficiently, however, the fetus struggles to get the nutrients and, especially, the oxygen it needs. Growth slows, and the brain begins running short on oxygen. The panicked fetus and the placenta throw a massive tantrum to get what they need.
Like a baby crying for milk, the placenta signals the mother’s body to raise her blood pressure. The higher pressure forces more oxygen and nutrients through the placenta to meet the growing baby’s needs. But if the mother’s blood pressure climbs too high, it risks damaging the delicate blood vessels that bathe the placenta in blood as well as the fine capillaries in the mother’s kidneys, liver, lungs, and brain.
Damage to placental blood vessels means less oxygen gets through to the fetus, leading to more urgent demands for higher blood pressure. On top of that, the placenta starts to die, and dying placental cells get into the mother’s blood vessels, clogging them up and making the problem even worse. This vicious cycle, left untreated, can damage the mother’s organs, leading on to fits of eclampsia and a risk of brain damage and death.
The consequences for the baby can be just as severe as they are for the mother. Reduced blood flow and placental damage slow the fetus’ growth so drastically that babies of pre-eclamptic pregnancies tend to be born desperately small.
Fortunately, my friend Amy’s doctors delivered baby Simone, brought Amy’s convulsions under control and lowered her blood pressure enough to avoid permanent organ damage. But for a cautious GP, a quick-thinking obstetrician, and a pair of forceps, Amy, Simone, or both might well have died that day.
Despite her traumatic introduction to motherhood, Amy walked out of hospital within days, carrying baby Simone. The battle that had caused pre-eclampsia had slowed Simone’s growth, leaving her so small that even the tiniest baby clothes were too big for her when she was born. Luckily she grew quickly, escaping the many problems that accompany low birth weight. By one year of age, she had attained average weight and height, and she grew into a robustly healthy, delightful young woman who excelled at school and on the sports field.
Postscript: Welcome to the world of sexual conflict
In this rather long and technical story, I have considered how a perplexingly complex condition — pre-eclampsia — might arise through conflict between a mother and her developing fetus. The very different evolutionary interests of the fetus’ mother and father, and especially of the genes the fetus inherited from each parent, sharpen this conflict.
In a fair and just world designed by an omniscient creator, the genes a fetus inherits from mum and dad would quietly get on with their important shared task of making that fetus into a happy, healthy baby that doesn’t cause its mother too much pain on the way out. Instead, the genes bicker and argue, with those inherited from dad trying to take the mother for every molecule of glucose and oxygen the fetus can get. The genes inherited from the mother defend her so she can have more babies later on.
A detailed look at the hormones, cells, and genes involved in pregnancy reveals a story at profound odds with the Hollywood script and the peppy tone of “what-to-expect” pregnancy bestsellers. While pregnancy certainly imbues some expectant mothers with a warm inner glow, within the womb there rages a mighty battle.
In coming articles, I will show how that battle’s rage is felt beyond the panic of pre-eclampsia, in the nausea of pregnancy sickness and the glucose highs of gestational diabetes. And I will reveal some big twists in the pre-eclampsia tale that might give wannabe parents a reason to have a lot more sex.
If the genes a fetus inherits from its parents can’t get along, then what hope is there for the parents themselves? Or for men and women in general? Those questions animate much of my writing, here and elsewhere.



